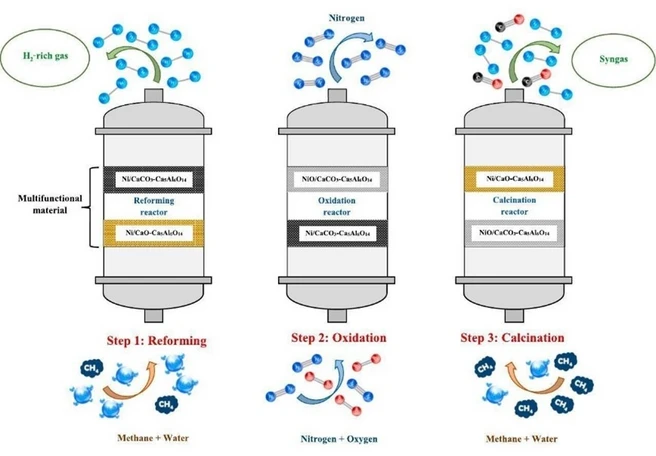
The team develops an integrated SE-SMR-CL process (sorption-enhanced steam methane reforming - chemical looping) that couples reforming, oxidation, and calcination and uses the released CO₂ in-situ during the regeneration step. Thermodynamic analysis shows up to 93.84% H₂ purity at 650 °C and 1 bar for a steam to carbon molar ratio (S/C) of 3) in the reforming step, and H₂/CO ≈ 1.58 at 850 °C during calcination (CH₄/CaCO₃ = 2); steam addition can further increase the H₂/CO ratio depending on the amount. As a proof of concept, a multifunctional Ni/CaO–Ca₅Al₆O₁₄ material was employed: experimentally, at 650 °C, 1 bar, S/C = 3 and WHSV = 1800 mL h⁻¹ gcat⁻¹, about 80 vol% H₂ was achieved; at 850 °C, syngas with H₂/CO ≈ 1 is formed. Steam addition markedly suppresses coking and typically increases the syngas quality to about 1.2–1.4. In addition, chemical-looping oxidation reduces the external heat demand; calcination at 850 °C is sufficient to achieve high CO₂ conversion without carbon deposition. Performance remains stable for at least five cycles, the Ca₅Al₆O₁₄ phase acts as a support, mitigates sintering, and stabilizes the CO₂ uptake of CaO. The resulting H₂/CO mixtures are direct precursors for methanol, DME, or Fischer-Tropsch synthesis. Overall, the study shows how existing SMR technology can be environmentally improved and economically upgraded through simultaneous, quality-adjustable co-production of H₂ and syngas.
[1] N. Hemsap, et al. “In-situ CO2 utilization for dual production of hydrogen-rich gas and syngas via sorption-enhanced steam methane reforming chemical looping.” Chemical Engineering Journal 509 (2025) 161127.